Snowwhite2 paper: Selective laser sintering of distinct drug and polymer layers
In this installment of our series showcasing the impact of the Snowwhite SLS 3D printer on scientific advancement, we’re focusing on the paper “Selective laser sintering of distinct drug and polymer layers as a novel manufacturing strategy for individually dosed tablets“. We’ll kick things off by breaking down the study’s purpose and its key outcomes in plain language, ensuring everyone can grasp the significance of the research. Then, for those interested in the technical details, we’ll present the original abstract and any associated references.
Understanding the study and its main result
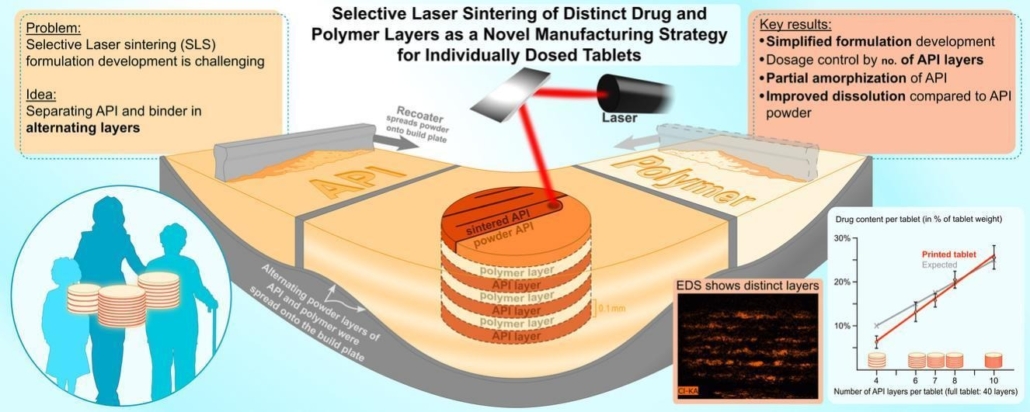
This study looked into a new way to make personalized medicine using our Sharebot Snowwhite2 SLS printer. Instead of mixing drug powder and other ingredients together, they used separate containers for the drug (indomethacin, or IND) and another material (polyvinyl alcohol, or PVA). This allowed them to print alternating layers of pure drug and pure excipient in one go.
They successfully made tablets with different doses of IND just by changing the number of drug layers. A big achievement was being able to print pure IND, which is usually hard to print by itself. They also found that the printing process changed the drug slightly, which might help it dissolve and work better in the body. Tests showed that the printed tablets dissolved faster than regular IND powder.
Main result
The main discovery is a simplified and effective method for creating personalized, multi-layered drug tablets using Selective Laser Sintering (SLS) without needing to pre-mix powders. This approach allows for direct printing of distinct drug and excipient layers, enabling precise dose control and potentially improving drug absorption. A key part of this discovery was successfully printing pure crystalline indomethacin, which was previously thought to be very difficult with SLS technology.
Selective laser sintering of distinct drug and polymer layers as a novel manufacturing strategy for individually dosed tablets
Jonas Autenrieth (a), Daniel Hedbom (b), Maria Strømme (b), Thomas Kipping (c), Jonas Lindh (b), Julian Quodbach (d)
a) Division of Molecular Pharmaceutics, Department of Pharmacy, Uppsala University, Uppsala Biomedical Center, P.O Box 580, SE-751 23 Uppsala, Sweden
b) Division of Nanotechnology and Functional Materials, Department of Materials Science and Engineering, Uppsala University, Ångström Laboratory, Regementsvägen 1, Uppsala 751 03, Sweden
c) Merck Life Science KGaA, Frankfurter Str. 250, Postcode: D033/001, DE-642 93 Darmstadt, Germany
d) Department of Pharmaceutics, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Universiteitsweg 99, 3584 CG Utrecht, the Netherlands
Ref.: https://www.sciencedirect.com/science/article/pii/S2590156725000234
Abstract
Selective Laser Sintering (SLS) is an emerging additive manufacturing technology with potential for the production of personalized pharmaceuticals. In this study, we investigated a novel simplified formulation approach in SLS-based manufacturing of individually dosed, multi-layered tablets with distinct layers of pure active pharmaceutical ingredient (API) and excipient. Indomethacin (IND) was chosen as the model API, and polyvinyl alcohol (PVA) served as the excipient. Unlike conventional methods requiring powder blending, this approach utilizes separate powder tanks for IND and PVA, enabling direct printing of alternating layers in a single-step procedure.
We successfully fabricated tablets with controlled IND doses by varying the number of IND layers, maintaining consistent printing parameters across different compositions and confirming the API’s chemical stability in the product. Since SLS is conventionally used for thermoplastic substances, the successful sintering of pure IND layers was a key achievement in the study, as this crystalline API is typically not printable separately. Energy dispersive X-ray spectroscopy (EDS) demonstrated the successful formation of distinct API and excipient layers. Differential scanning calorimetry (DSC) characterization revealed that the sintering process partially amorphized IND, which may enhance dissolution and bioavailability. Dissolution testing indicated that the printed tablets exhibited improved dissolution rates compared to raw IND powder.
The study successfully demonstrated the possibility of SLS-based production for personalized dosing by omitting powder blending steps. The ability to create individualized dosages with minimal excipients and simplified processing represents a step toward further investigation of SLS for clinical settings, including hospital and pharmacy-based drug production.